Moderna Vaccine Efficacy
Vaccine efficacy was defined as the percentage reduction in the hazard ratio for the primary end point mRNA-1273 vs. US biotech company Moderna announced Covid vaccine is 90 percent effective against all forms of the disease and 95 percent effective against severe.

Covid 19 What Does Vaccine Efficacy Really Mean Diu News
Modernas COVID-19 vaccine have revealed 941 per cent efficacy researchers said.
Moderna vaccine efficacy. That number jumped to 90 two weeks. The same Canadian study that found the Pfizer-BioNTech vaccine to be 87 percent effective suggested that the Moderna vaccine was 72 percent effective against the Delta variant after one dose. Safety outcomes included adverse events and cases of COVID-19 and severe COVID-19.
The Pfizer-BioNTech vaccine was said to be 95 effective. It is being widely used in the United Kingdom Canada the European Union the United States and Singapore where it has either regulatory approval or emergency authorisation. Moderna Inc MRNAO said on Thursday its COVID-19 shot was about 93 effective through six months after the second dose showing hardly any change from the 94 efficacy reported in.
The mRNA-1273 vaccine showed 941 efficacy at preventing Covid-19 illness including severe disease. Moderna specified efficacy outcomes as primary and secondary endpoints with the primary endpoint being efficacy of the vaccine in preventing symptomatic infection at least 14 days after the second injection of vaccine. Aside from transient local and systemic reactions no safety concerns were identified.
The Food and Drug Administrations staff said in a report on Tuesday that the experimental. In clinical trials Modernas vaccine reported 941 effectiveness at preventing COVID-19 in people who received both doses. Modernas two-dose vaccine lasts through six months.
Funded by the Biomedical Advanced Research and Development Authority and the. Drugmaker Moderna announced Wednesday that its COVID-19 vaccine is 93 effective after six months. August 25 2021 746 AM 1 min read.
Antibody levels are a good predictor for how effective Moderna Incs COVID-19 vaccine is according to a new study released on Tuesday a finding which could help speed up future clinical trials for vaccines against the disease. Moderna Incs vaccine is safe and effective for preventing Covid-19 in people ages 18 and older US regulators said clearing the way for a second shot to quickly gain emergency authorisation and add to the countrys sprawling immunisation effort. Results from the primary analysis of the ongoing phase 3 clinical trial of US biotechnology company Modernas Covid-19 vaccine have revealed 941 per cent efficacy of.
The Moderna vaccine has been found to have strong efficacy in preventing symptomatic COVID-19 and severe COVID-19 in clinical trials. A new CDC study reported that a single dose of Pfizers or Modernas COVID vaccine was 80 effective in preventing infections. A large 2020 trial involving 30420 adult volunteers at various sites across the US reports that the Moderna vaccine has a 941 efficacy rate against COVID-19 including against severe disease.
MRNA a biotechnology company pioneering messenger RNA mRNA therapeutics and vaccines to create a new generation of transformative medicines for patients today announced that the primary efficacy analysis of the Phase 3 study of mRNA-1273 conducted on 196 cases confirms the high efficacy observed at the first interim. According to the company a final analysis of its Phase 3 trials found the vaccine was 93 effective and that efficacy holds strong for. The study which has not yet been peer-reviewed found that the Moderna vaccine was more effective in vaccine recipients with high levels of antibodies.
Moderna also said Thursday a final analysis of its phase three study found the two-dose vaccine was 93 effective with efficacy remaining durable through six months after the second dose. The Moderna news followed preliminary results from the Pfizer-BioNTech vaccine candidate BNT162b2 with Moderna demonstrating similar efficacy but requiring storage at the temperature of a standard medical refrigerator of 28 C 3646 F for up to thirty days or 20 C 4 F for up to four months whereas the Pfizer-BioNTech. Modernas Covid-19 vaccine shows 945 efficacy against virus says company The drug manufacturers Chief Medical Officer Tal Zaks said the overall effectiveness of their vaccine was.
Results from the primary analysis of the ongoing phase 3. A stratified Cox proportional hazards model was used to.

Safety And Efficacy Of The Chadox1 Ncov 19 Vaccine Azd1222 Against Sars Cov 2 An Interim Analysis Of Four Randomised Controlled Trials In Brazil South Africa And The Uk The Lancet

Are Vaccines Becoming Less Effective At Preventing Covid Infection Financial Times

Pfizer Vs Moderna Vs Astrazeneca Whose Efficacy To Trust Businesstoday

Are Vaccines Becoming Less Effective At Preventing Covid Infection Financial Times
A Marm Kilpatrick On Twitter Vaccine Efficacy In Blocking Infection Transmission I Think We Can Now Estimate The Minimum Reduction In Transmission From The Moderna Vaccine Thread Tl Dr Moderna Vaccine Blocks
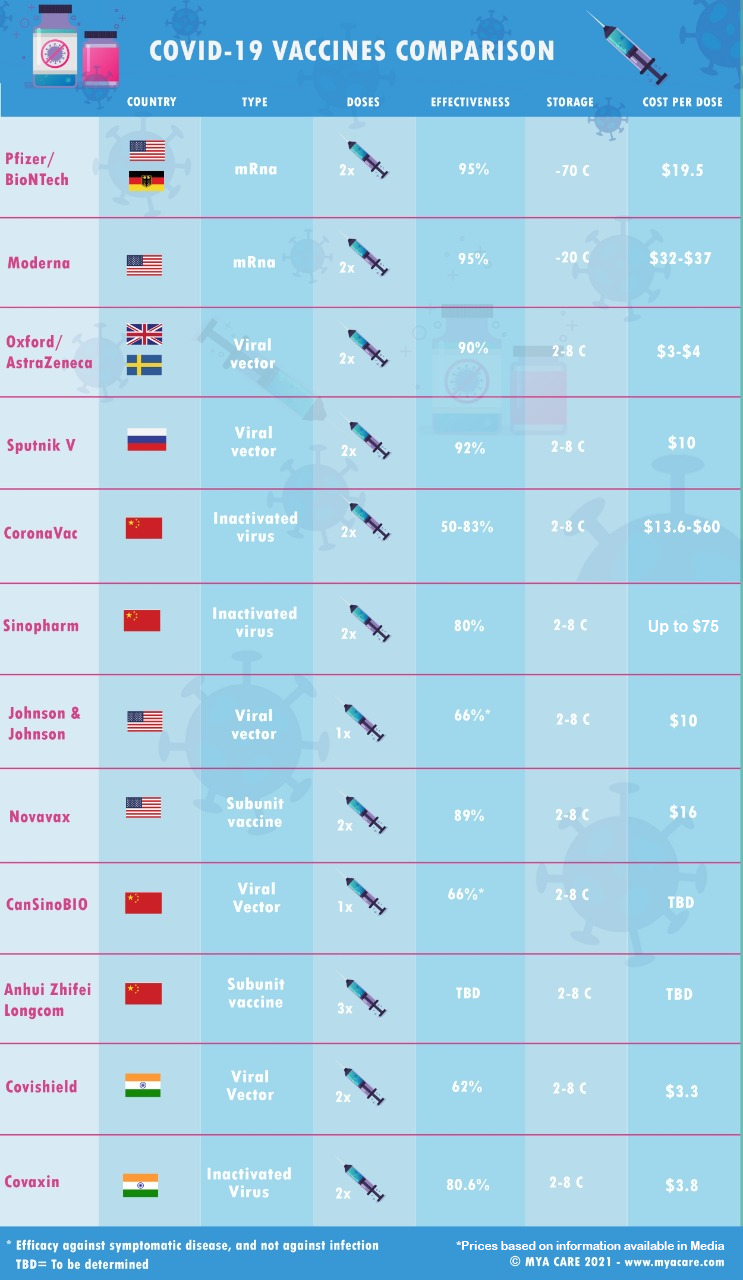
Comparison Of Covid 19 Vaccines Mya Care
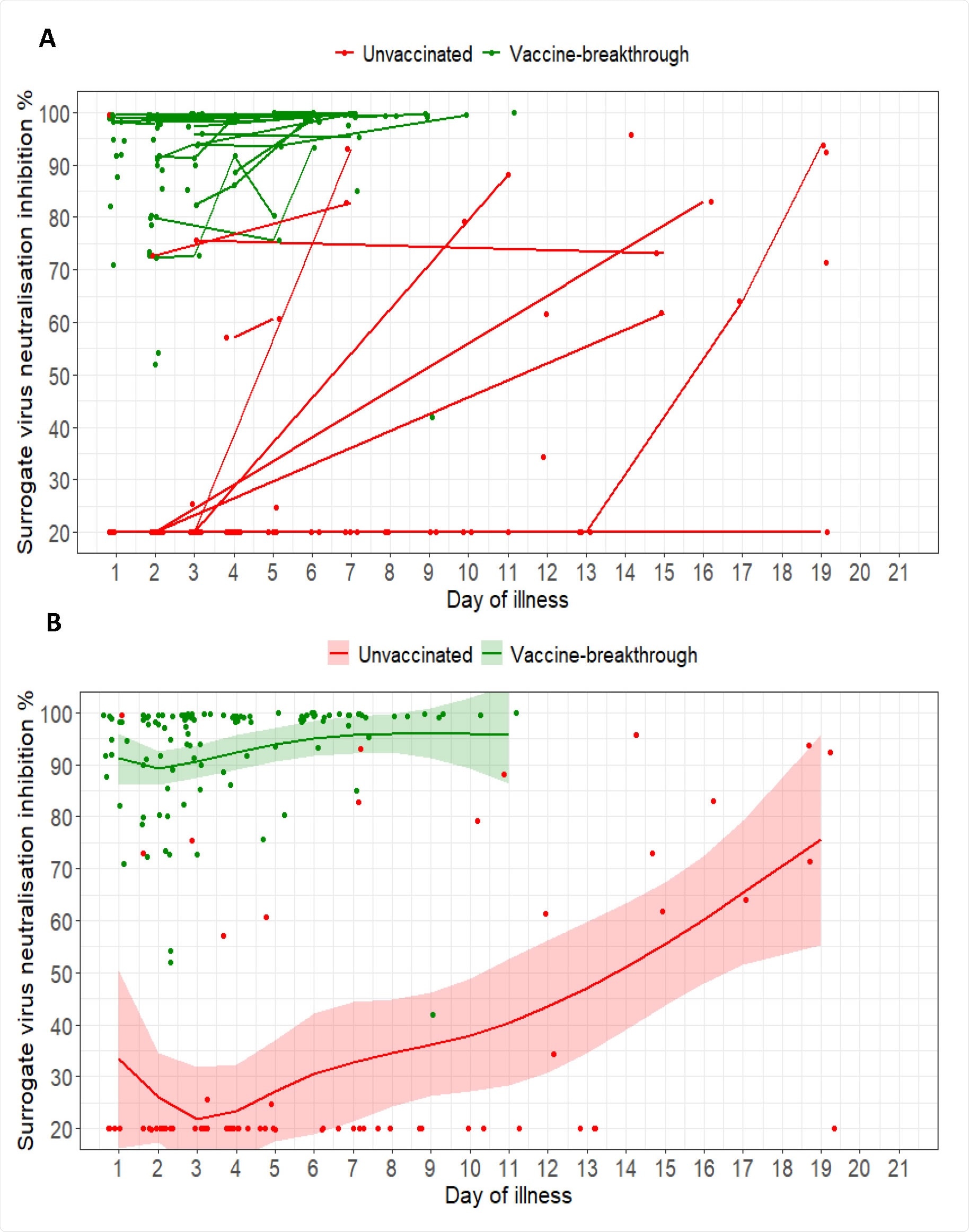
Study Shows Efficacy Of Pfizer Biontech And Moderna Vaccines Against Sars Cov 2 Delta Variant

Moderna Covid Vaccine Efficacy Drops As Trial Subjects Double Businesstoday

Covid 19 What Does Vaccine Efficacy Really Mean Diu News
Coronavirus Vaccine Efficacy Compared To Shots For Other Viruses
Comparing Vaccines Efficacy Safety And Side Effects Healthy Debate
Posting Komentar untuk "Moderna Vaccine Efficacy"