Moderna Vaccine Journal
Published in the journal Science. FRANCE KEYSER for The Wall Street Journal.
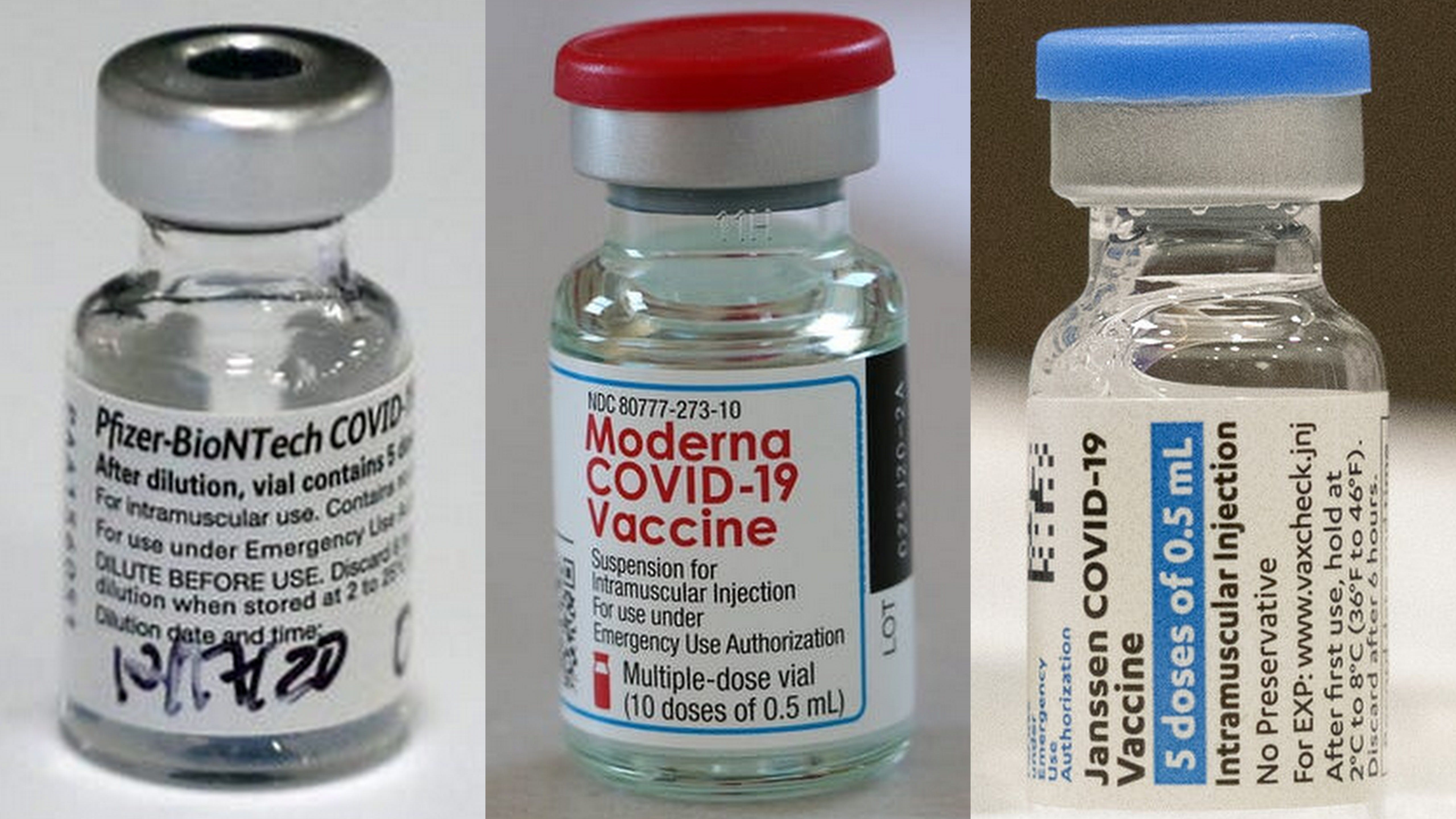
Take Your Best Shot At Convincing Folks To Be Vaccinated Against Covid
This article is in your queue.
Moderna vaccine journal. Modernas Covid-19 vaccine protects people for at least six months and likely longer -- even against new variants researchers reported Thursday. The SARS-CoV-2 pandemic continues to be a global health concern. FOREIGN Affairs Secretary Teodoro Locsin Jr.
This pruritic and variably tender reaction has a median duration of 5 days but may persist for up to 21 days and may occur again and sooner after the second vaccine dose. By Ramola D Source. NORWOOD Mass Moderna Inc.
Now a study in the journal Nature provides some hopeful news that these mRNA vaccines may be protective even longer 1. Investigators identified no safety concerns and no evidence of vaccine. The coronavirus disease 2019 Covid-19 pandemic is an international.
The foreign substance detected in some vials of Modernas Covid-19 vaccine in Japan was stainless steel and it isnt believed to affect the vaccines safety or efficacy Moderna and its. Elie Dolgin is a science. The vaccine also demonstrated efficacy in preventing severe COVID-19.
Results hint that dose stretching could help to address the worlds acute vaccine shortage. Real-world evaluations have demonstrated high effectiveness of vaccines against COVID-19associated hospitalizations 14. Immunogenicity and the mRNA-1273 SARS-CoV-2 Vaccine Thirty-four adults received two 100-μg injections of the mRNA-1273 SARS-CoV-2 vaccine and serum antispike protein and neutralizing antibody.
Is adding two new production lines at the rebuilt former Polaroid plant where it manufactures its Covid-19 vaccine. Earlier this year clinical trials of the Moderna and Pfizer-BioNTech vaccines indicated that both immunizations appeared to protect for at least six months. Tuesday December 22 2020.
Yesterday said negotiations for coronavirus vaccine supply with American biotechnology company Moderna will begin on or before December 30. American Medical Researchers Witness SELF-ASSEMBLING Graphene Oxide Nanotech or AI Syn Bio in Moderna Vaccine Under Microscope. Ramola Ds report with Everyday Concerned Citizen.
The investigational vaccine known as mRNA-1273 was 941 efficacious in preventing symptomatic coronavirus disease 2019 COVID-19 according to preliminary results from a Phase 3 clinical trial reported in the New England Journal of Medicine. Said Monday that it will supply 34 million doses of its Covid-19 vaccine to an international program that distributes free shots to poorer countries. A breakthrough coronavirus vaccine could come in trials starting this month.
The mRNA-1273 Moderna vaccine was reported to have an efficacy of 941 at preventing symptomatic COVID-19 due to infection with. In bombshell news pointing to the much-speculated-on presence of nanobots in vaccines an American medical researcher reports that moving shifting self. Moderna vaccine purchase pushed.
Evaluations of authorized mRNA COVID-19 vaccines Pfizer-BioNTech and Moderna have consistently demonstrated high VE across diverse populations 15Because COVID-19 vaccines were initially authorized in the United States in December 2020. MRNA has shifted gears on its own company vaccine policy mandating Covid-19 vaccines for all US. QUICK TAKE Phase 1 Study of a SARS-CoV-2 mRNA Vaccine in Older Adults 0204.
The doses will be delivered in. Monday December 21 2020. Stéphane Bancel the CEO of Moderna in Marseille France this week.
Findings The Moderna COVID-19 vaccine may cause a delayed localized hypersensitivity reaction with a median latency to onset of 7 days after vaccine administration. Nonhuman primates received 10 or 100 μg of mRNA-1273 a vaccine encoding the prefusion-stabilized spike protein of SARS-CoV-2 or no vaccine. A two-dose regimen of BNT162b2 30 μg per dose given 21 days apart was found to be safe and 95 effective against Covid-19.
Persistence of Antibody after mRNA-1273 Vaccination A total of 33 participants who received both doses of the Moderna mRNA-1273 vaccine against SARS-CoV-2 had blood drawn over a period of 6 months. Quarter-dose of Moderna COVID vaccine still rouses a big immune response.
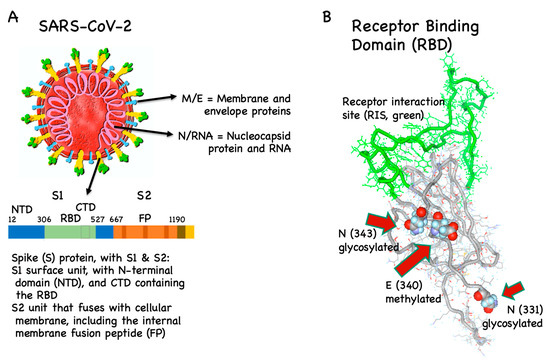
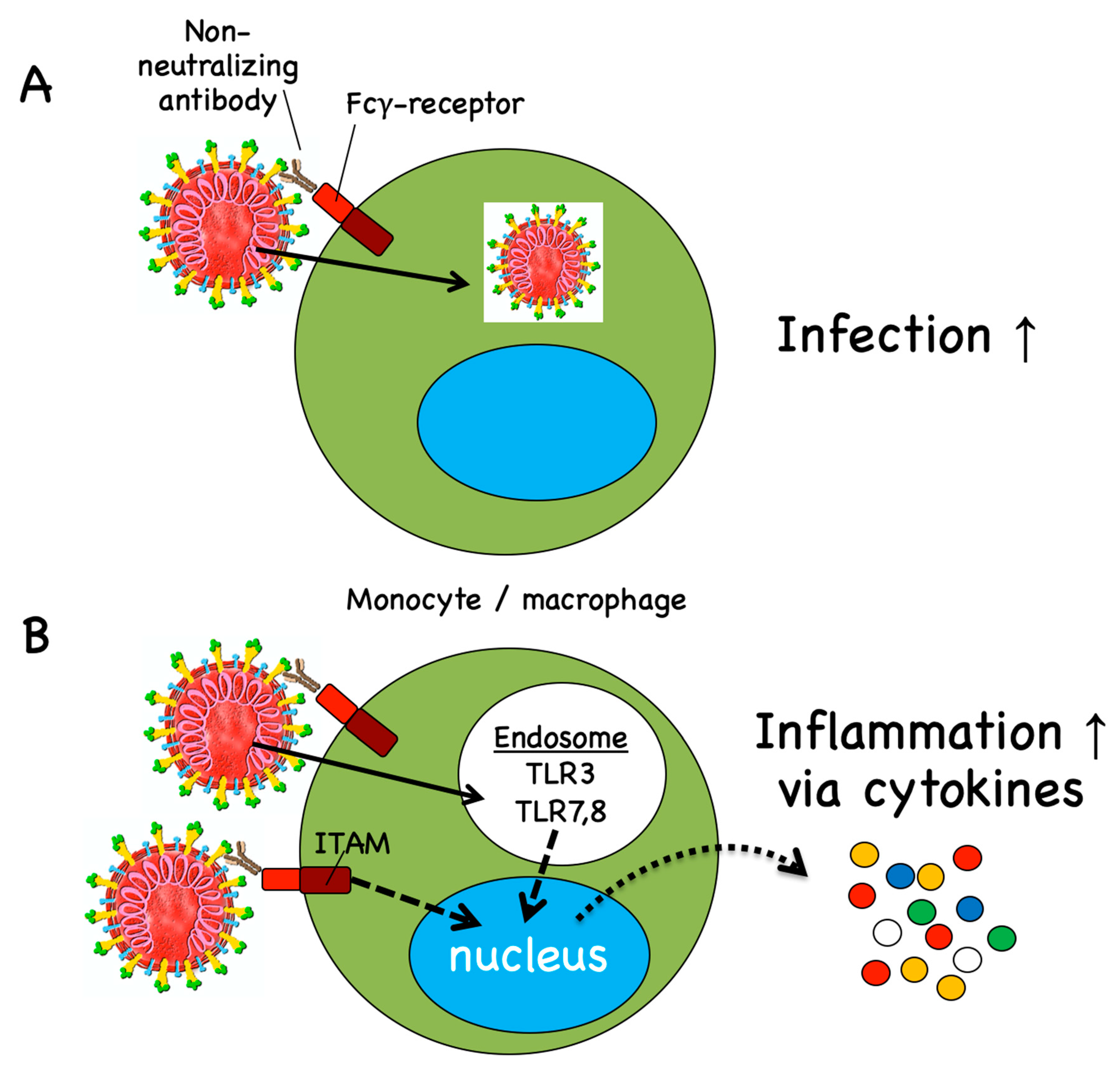
Vaccines Free Full Text Covid 19 Mechanisms Of Vaccination And Immunity Html
Moderna Covid Vaccine Has 94 Efficacy Final Results Confirm Coronavirus The Guardian

No Sign Of Pfizer Moderna Covid 19 Vaccines In Breast Milk Study Shows

Moderna Vaccine Creates Twice As Many Antibodies As Pfizer Study Shows The Boston Globe
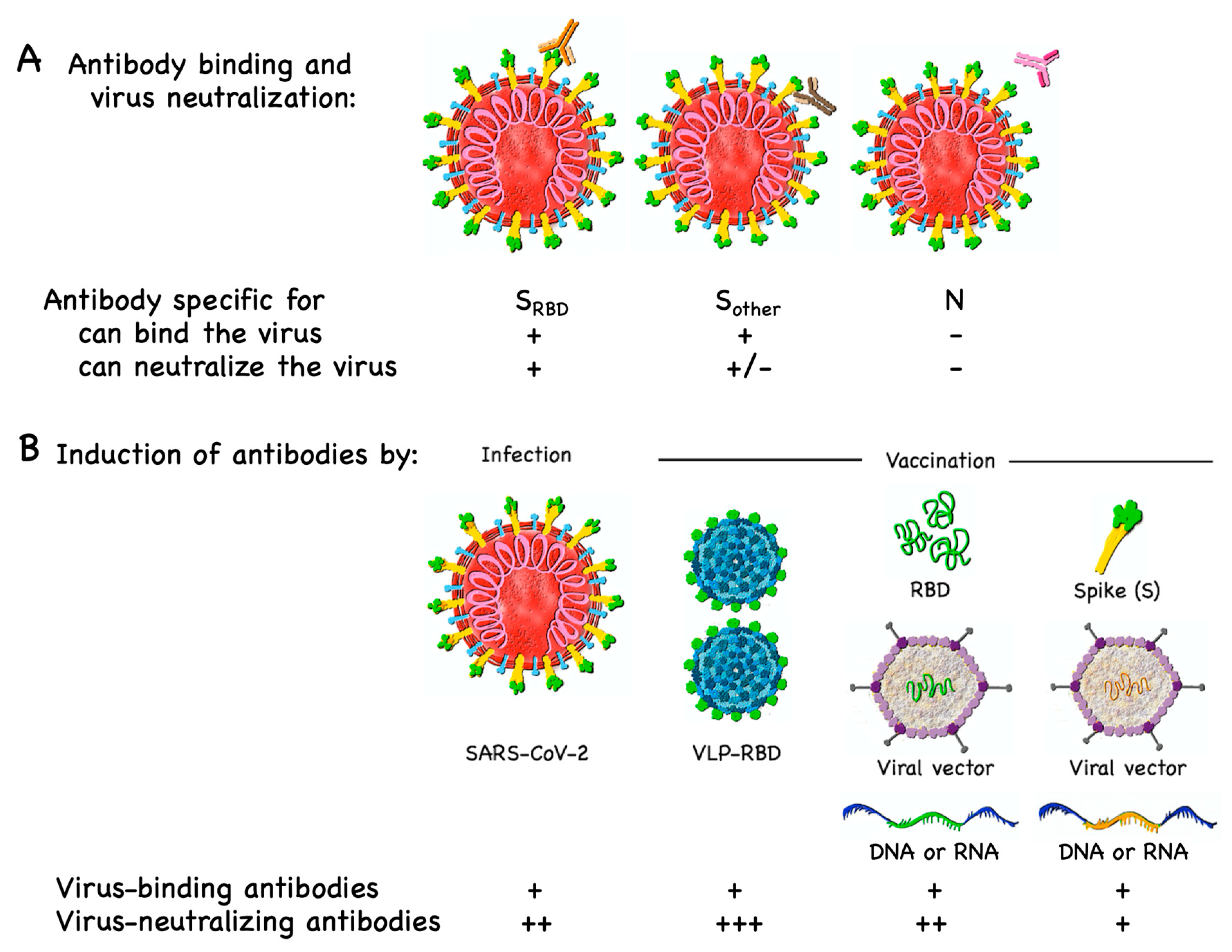
Vaccines Free Full Text Covid 19 Mechanisms Of Vaccination And Immunity Html

Vaccines Free Full Text Covid 19 Mechanisms Of Vaccination And Immunity Html

Cutaneous Reactions Reported After Moderna And Pfizer Covid 19 Vaccination A Registry Based Study Of 414 Cases Journal Of The American Academy Of Dermatology
Covid Arm Skin Reactions At Injection Site Of Moderna Vaccine In Bc Case Reports British Columbia Medical Journal

Moderna Covid 19 Vaccine 93 Effective Durable At 6 Months Company Says

Covid 19 Safety And Efficacy Of The Mrna 1273 Sarscov 2 Vaccine Moderna Against Sars Cov 2

Covid 19 Safety And Efficacy Of The Mrna 1273 Sarscov 2 Vaccine Moderna Against Sars Cov 2
Posting Komentar untuk "Moderna Vaccine Journal"